Levagen+™ is a clinical compound of palmitoylethanolamide (PEA), an endogenous fatty acid amide belonging to the endocannabinoid family. PEA was first identified in 1957 in egg yolks, soybeans and peanuts. It is an active lipid that acts on the cannabinoid receptor CR2 to treat joint pain and injury and relieve inflammation.
Standard PEA does not dissolve well in water, which can limit its effectiveness. Levagen+™ is a cold water dispersible PEA powered by Pharmarko Biotechnologies’ Lipisperse® technology to increase bioavailability and functionality in aqueous environments such as the stomach. In aqueous environments, Lipisperse® enhanced active particles can freely disperse, which help enhance its absorption in the body’s natural digestive process.
While PEA is commonly used to treat pain and injury in athletes and the ageing population, it is not well known that it can also be used as a support for the flu and common cold. Multiple clinical trials were conducted on nearly 4000 people and published in former Czechslovakia between 1969-1979, with all studies demonstrating its efficacy in treating and alleviating symptoms of the flu and common cold.
History of PEA
PEA was first marketed as 300mg tablets in the 1960s as a treatment and prophylactic for the flu and common cold.
Five double-blind, placebo-controlled studies were conducted in adults between 1969 – 1979 during influenza season. A final one was conducted in 1977 in children.
Trial Results
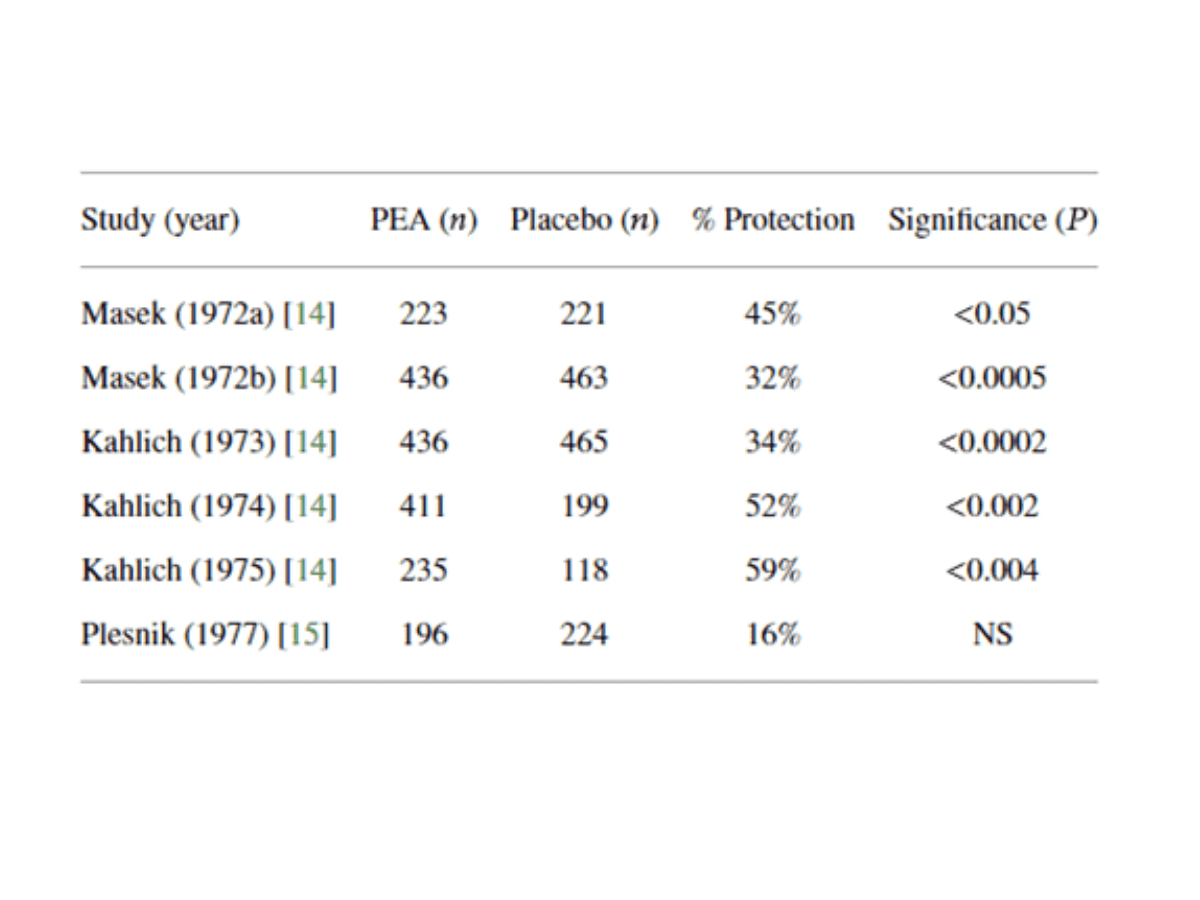
The first trial of 444 subjects tested PEA as a treatment for cold and flu symptoms. Subjects had to register symptoms of headaches, sore throats, temperatures above 37.5°C, dry coughs, fatigue and nasal discharge. Subjects were required to take 600mg of PEA three times daily for 12 days. The results showed that the group taking PEA had a 45.5% reduction in fever and pain when compared to the group taking placebos.
The second trial tested PEA’s ability to prevent the flu and common cold. The subjects were made up of 899 healthy volunteers from an army unit taking 600mg of PEA three times daily for three weeks, followed by one 600mg dose once daily for six more weeks. The results showed that by week six, the group taking PEA’s incidence of disease was 40% lower, then 32% lower at week eight when compared to the group taking placebos.
Three more trials were conducted in army soldiers with a total of 678 subjects to test the manifestation rate of influenza and the common cold. In all three studies, all groups taking PEA had significantly lower rates of manifestation than the groups taking placebos.
In all trials, no side effects were recorded. Kahlich et al., who conducted the studies, observed that PEA had “clear treatment effects in respiratory infections” and “the ease of application of PEA offers the possibility to have a quick therapeutic answer ready in case of a flu epidemic”.
How does PEA work for Anti-influenza and the Common Cold?
When the influenza virus enters the body, the immune system fights it by releasing pro-inflammatory proteins called cytokines, which has antiviral properties by inducing symptoms such as high temperatures, nasal discharge and fevers.
A cytokine storm occurs when the immune system overproduces cytokines, which is linked to severe inflammation, disease and mortality in people infected with the influenza virus with otherwise healthy immune systems.
PEA’s anti-inflammatory properties, which is also effective for joint and pain relief, works for anti-influenza and the common cold relief by inhibiting some cytokine production which appears to help decrease the symptoms of flu and the common cold.
The Study’s Conclusion
Hesselink et al. suggest that given the results of the clinical trials, PEA should be considered as a treatment for influenza virus and respiratory infections. PEA has low chances of resistance, unlike common pharmaceutical antiviral drugs.

PEA as anti-inflammatory properties which can help decrease the symptoms of flu and common cold.
What this means for us
With Levagen+™’s enhanced form of PEA powered with Lipisperse® technology, this gives vitamin and supplement companies have the opportunity to expand their immunity product range to provide their customers with more options. Based on the clinical trials, PEA had no side effects, showed its efficacy in reducing symptoms of the flu and common cold, and lowered chances of manifestation rates. It is a food component and produced in the body, making it a natural alternative instead of antiviral drugs which can have resistance. Levagen+™’s PEA is manufactured with the highest biotechnology available to ensure its efficacy and potency when compared to PEA found in food sources.
If you enjoyed reading this blog, consider joining our mailing list to ensure you are up to date with the latest health and complementary medicine news and information.
Lipa Pharmaceuticals is proud to use Levagen as one of our key materials.
Reference List
J.M. Keppel Hesselink, Tineke de Boer, Renger F. Witkamp. Palmitoylethanolamide: A Natural Body-Own Anti-Inflammatory Agent, Effective and Safe against Influenza and Common Cold. International Journal of Inflammation Volume 2013 (2013), Article ID 151028, 8 pages, http://dx.doi.org/10.1155/2013/151028.
Work with Australia’s Leading Pharmaceutical Manufacturer
Businesses seeking an alternative pharmaceutical manufacturer in Australia need look no further than Lipa Pharmaceuticals. We are an 8-time winner of Australia’s High Quality Manufacturer Award for complementary medicines and have the capability to manufacture high volumes of vitamins, health food supplements, and over-the-counter medication.
With over 30 years of industry experience and fully-equipped production facilities, we can work with your business to produce custom products in a private label or contract manufacturing arrangement. Get in touch with us today to find out how to partner with our cost-effective pharmaceutical manufacturing solutions in Australia.
